FDA annouces CDRH user fees for Fiscal 2024
- 14 August 2023
- Posted by: inetika
- Category: GLOBAL NEWS
No Comments
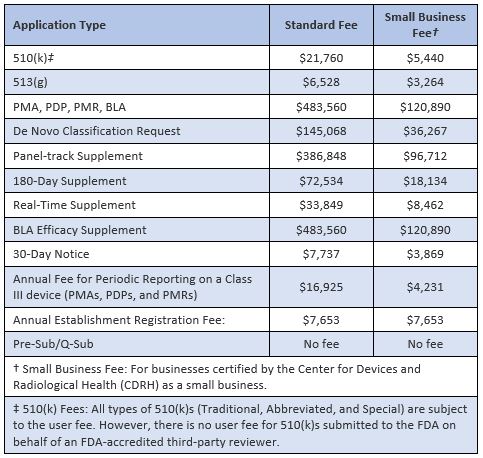
The US Food and Drug Administration (FDA) has announced the user fees that will be payable by medical device manufacturers for interactions with the Agency’s Center for Devices and Radiological Health (CDRH) during Fiscal Year 2024, starting on 1 October 2023. The new fees, which include an increase of around 7.6% over the previous year, are shown in the table below:
Further details are available from here.

